Joint Nordic HTA-Bodies
The Nordic collaboration JNHB offers efficient and transparent joint health technology assessments of medicinal products in the five Nordic countries.
The Joint Nordic HTA Bodies – JNHB
The Nordic collaboration JNHB offers efficient and transparent joint health technology assessments of medicinal products in the five Nordic countries.
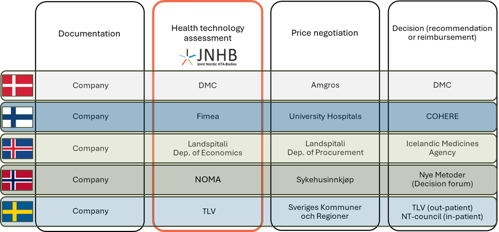
The collaborating HTA bodies are the Danish Medicines Council (DMC), the Finnish Medicines Agency (Fimea), Landspitali- The National University Hospital of Iceland, the Norwegian Medical Products Agency (NOMA) and the Dental and Pharmaceutical Benefits Agency (TLV) in Sweden.
A JNHB assessment would ideally add only two new features to the current national processes:
- The health technology developer (HTD) signs the JNHB Waiver of Confidentiality to allow HTA bodies to share information during the assessment, and to share the unredacted JNHB assessment report with the price negotiators and decision makers in respective country.
- The HTD submits the dossier and health economic model to TLV, that distributes the material to the JNHB assessment team.
Joint assessments of pharmaceutical products include both relative effectiveness and health economics. The assessment reports are designed to support the decision processes in the five countries, according to the legal requirements and procedures of each country. The joint HTA assessment allows the HTA bodies to share the different tasks of the assessments; this leads to higher joint quality and more time efficient procedures. JNHB collaboration is not aiming for joint decisions on recommendations or reimbursement.
JNHB and introduction of new medicinal products in the Nordic countries
Each Nordic country has its own framework for pricing and decision making when introducing new medicinal products. JNHB supports the national processes by providing joint health technology assessments (HTAs), ensuring consistent and evidence-based evaluations across Denmark, Finland, Iceland, Norway, and Sweden. Pricing and recommendation/reimbursement decisions remain national responsibilities.
The figure below gives an overview of the organizations involved in HTA, negotiations and decision-making in the Nordic countries. In Denmark, Finland and Norway, out-patient pharmaceuticals have separate processes that are not included in the figure.
Find the fact sheet below and read more:
JNHB and introduction of new medicinal products in the Nordic countries
There are several advantages for joint Nordic assessments of medicinal products.
Joint Nordic HTA ensures that the Nordic countries assess the medicine simultaneously, using the same evidence base when conducting an assessment. By working together and sharing knowledge, we aim at producing high quality assessments reports providing a solid support for the national decisions on reimbursement and recommendation in the Nordic countries.
The JNHB collaboration aims at:
• Supporting timely and equal access to medicinal products for patients in the Nordic countries
• Less divergence in HTA methodologies and evidence requirements between the Nordic HTA bodies
• Sharing of resources and knowledge between the Nordic HTA bodies
• Increased efficiency in generation of assessment reports
• Reduced administrative burden for industry
Joint Nordic assessment is one factor among others that could facilitate equal access to medicines in the Nordic countries, and a JNHB assessment can facilitate Nordic price negotiations for relevant products. However, the decisions on reimbursement and recommendation are national decisions made in each of the Nordic countries.
An advantage for companies is that the application will be handled efficiently and simultaneously with one point of contact instead of multiple parallel processes with different demands and requirements along the way.
Memorandum of Understanding
The terms of the cooperation are clarified in the Memorandum of Understanding, originally signed in September 2017 and renewed in June 2020. The memorandum was renewed again when Denmark joined the collaboration in May 2023 and when Iceland joined in April 2024.

