The Joint Nordic process
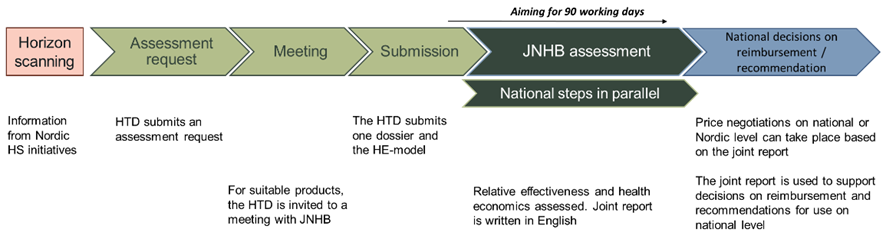
The main steps of the JNHB process are outlined in the figure above.
Find the JNHB process guideline below:
Horizon Scanning
JNHB collaborates with Nordic Horizon Scanning initiatives and receives information about which new medicinal products, and new indications for already approved products are on the way to the market. The information also includes facts about the products characteristics that facilitates when identifying products interesting for Nordic collaboration.
Assessment request
The health technology developer (HTD) is invited to submit a PICO (Population, Intervention, Comparator(s), Outcomes) for their product. To allow joint work with confidential material, the HTD is asked to submit the Waiver of confidentiality to JNHB at this timepoint. You can find the waiver of confidentiality below:
The JNHB team conducts a pre-assessment of the assessment request to determine whether the product is suitable for a joint JNHB assessment. This includes considering any national aspects that may need to be addressed and ensuring that the current treatment practices are sufficiently comparable in the Nordic countries. If the pre-assessment concludes that the product is suitable for a joint JNHB assessment, the HTD is invited to a meeting.
Meeting
The HTD is invited to a meeting to discuss timelines and submission. The meeting is also an arena/platform for any questions the HTD may have (e.g., differences between the national and joint assessment procedure).
Submission
The HTD submits their dossier with the required documents and health economic model. The information and documentation that is formally required for a JNHB submission to be considered as complete is found in the list of required documents in the JNHB submission dossier template. We recommend using the JNHB submission dossier template for the submission. Find the submission dossier template below:
For practical reasons, JNHB submissions are sent to TLV and should be submitted according to TLVs national procedure.
- For out-patient medicinal products TLVs national procedure should be used, Apply for reimbursement - Tandvårds- och läkemedelsförmånsverket TLV.
- For hospital medicinal products the submission is sent by e-mail to registrator@tlv.se.
If the HTD wishes to submit something that should not be shared between the HTA bodies, such as confidential price information, this information should be sent to the concerned country by e-mail, as descried in the Waiver of Confidentiality.
Assessment
The assessment starts when JNHB confirms that the submission package is complete. A contact person at one of the HTAb will coordinate the communication between the HTD and the assessment team throughout the assessment process. The HTA bodies write a draft joint JNHB report in English.
Before publication, the draft report is shared with the HTD to check for factual errors and information the HTD considers confidential.
The joint report will be complemented with national details in a national appendix when required and used for national decisions recommendation and reimbursement. The joint report can also be used for price negotiations on national or Nordic level.
Working with confidential information in the JNHB collaboration
By signing the JNHB Waiver of Confidentiality the health technology developer (HTD) allows the HTA bodies to share and discuss the submission during the joint assessment and to produce a joint assessment report based on both non-confidential and confidential information.
The joint report is used to support decisions on recommendation or reimbursement on national level. The report can also be used to support price negotiations on national or joint Nordic level. Therefore, the unredacted JNHB assessment report needs to be shared with the price negotiators and decision makers in respective country, who accordingly are included in the Waiver of Confidentiality.
In addition, the waiver includes persons deemed necessary by the parties, such as contracted experts and officials. The details are outlined in the Waiver of Confidentiality.
The HTD may have prices or specific information intended for use in only one country, e.g., information related to local pricing. In this case, the HTD has the possibility to not include this information in the common submission but send it separately to the country in question, clearly indicating that this information is not intended for sharing within JNHB.
Before the joint assessment report becomes public, the HTD has the possibility to indicate whether they wish any information to be redacted prior to publication. However, the final decision on what information is redacted before publication is made by the HTA bodies, based on national legislations on publicly available documents.
The Waiver of Confidentiality specifies what information is subject to the waiver, who is party to it and which activities are covered by it as well as the terms by which the waiver is to be signed and terminated.
Collaboration with Nordic Pharmaceutical Forum
To further strengthen the cooperation between the Nordic countries, JNHB has entered a collaboration with the New Expensive Drugs (NED) section of Nordic Pharmaceutical Forum. The collaboration aims to support joint Nordic negotiations for products assessed through JNHB.
The possibility of joint HTA and negotiations is offered as a voluntary route for suitable products and aims at equal patient access in the Nordic countries. The main steps of the process are outlined below.
Points of contact between JNHB and New Expensive Drugs (NED) throughout the HTA and negotiation processes.
NED is a working group in the Nordic Pharmaceutical Forum. The group consists of the price negotiation authorities, Amgros I/S in Denmark, Sykehusinnkjøp HF, divisjon legemidler (LIS) in Norway, Landspitali- The National University Hospital of Iceland, and the New Drugs council (NT-council) in Sweden. Read more about the JNHB - NED collaboration below.
Read more about Nordic Pharmaceutical Forum and their work with joint Nordic negotiations at Nordic Pharmaceutical Forum (External link).
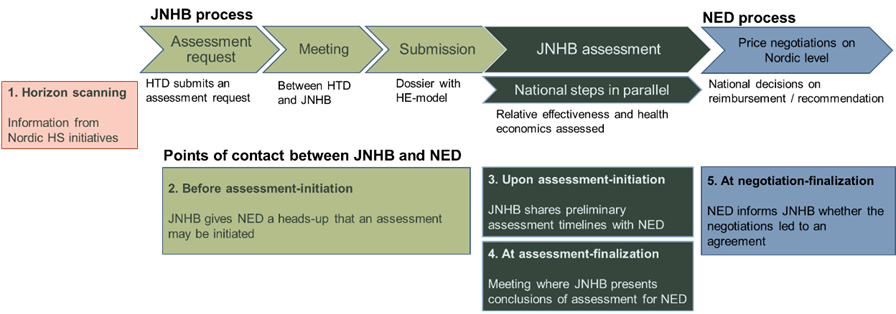
Points of contact between JNHB and New Expensive Drugs (NED) throughout
the health technology assessment (HTA) and negotiation processes are outlined in the figure above.
